Ozone Layer
Ozone is one of several gases that make up the earth's atmosphere. The diatomic molecule of oxygen (O2), the form of oxygen we breathe, makes up approximately 20 per cent of the atmosphere. Ozone (O3) the triatomic molecule of oxygen, is much less abundant, making up only one part in three million of all gases in the atmosphere.
Importance of Ozone
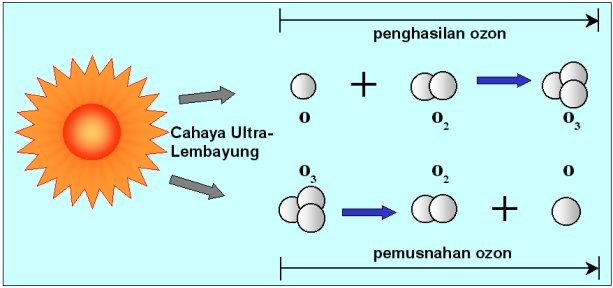
The ozone layer is concentrated within the stratosphere, between 20 to 40 kilometres from the surface of the Earth. Ozone can be produced by numerous chemical reactions, but the main mechanism in the atmosphere for its production and removal is absorption of ultra-violet (UV) radiant energy from the sun.
Ozone is produced when O2 absorbs UV radiation at wavelengths of less than 242 nanometres, and is removed by photo-dissociation from sunlight for wavelengths greater than 290nm. O3 is also the major absorber of UV sunlight between 200 and 330nm. The combination of these processes is effective in maintaining a relatively constant amount of ozone in the layer, and in absorbing 90% of UV sunlight.
Its primal function is to filter harmful ultraviolet (UV) radiation travelling from the sun to the surface of the Earth. The UV radiation if unfiltered can penetrate the protective coverings of flora and fauna, thereby causing severe damage genetically. This results in higher susceptibility towards pests and diseases for agricultural and forest ecosystems. Increased UV radiation in aquatic systems affect the nutrient mixing and thereby the aquatic life sustaining photosynthesis process.
The maintenance of enough stratospheric ozone to absorb harmful UV sunlight is therefore vitally important to all life forms on earth.
Ozone balance
Ozone measurements refer to the amount of ozone in a vertical column of air or a vertical ozone profile. This measurement is identified as the Total Ozone. The amount of ozone in the atmosphere varies according to geographic location and season. Ozone is scaled in Dobson units (Du), where, for example, 300 Du would be equivalent to a 3 mm thick pure layer of ozone if compressed to sea level pressure.
Stratospheric ozone is mainly produced in the tropics and transported to higher latitudes by large-scale atmospheric circulation during the winter to spring months. The tropics generally possess lower ozone.
Threat from Chloroflorocarbon (CFC)
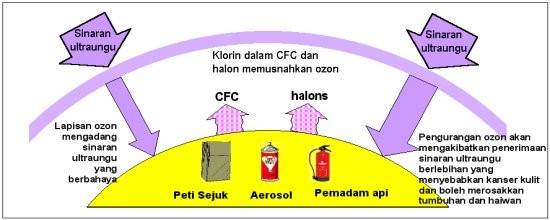
A known threat to this ozone balance is the introduction of man-made chlorofluorocarbons (CFCs) which increase the rate of ozone removal, resulting in a gradual decline in the global ozone levels.
CFCs are used by modern society in a myriad of ways, in refrigerants, as propellants in spray cans, in foam manufacture and in solvents, particularly for the electronics industry.
The long lifetimes of CFCs means that a single molecule released today can exist 50 to 100 years in the atmosphere before it is eliminated. Over periods of approximately five years, CFCs move slowly up into the stratosphere (10-50 km). Above the main body of the ozone layer, centred in the height range 20 to 25 km, less UV is absorbed by ozone. The CFC molecules break down after reaction with UV, and release free chlorine atoms. These chlorine atoms are then able to destroy ozone.
The Ozone 'Hole'
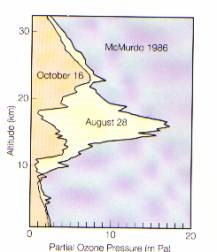
The Antarctic ozone 'hole' is caused by a springtime thinning of the ozone layer between particular altitudes over Antarctica. Such thinning has been detected each year for the past decade. Formation of the 'hole' occurs each September and recovery to 'normal' conditions occurs in late spring or early summer.
In October 1987, 1989, 1990 and 1991, deep ozone holes were observed over Antarctica with up to 60% less ozone compared to pre-ozone hole levels. In October 1991, the lowest levels of atmospheric ozone ever recorded occurred over Antarctica.
There appear to be two major reasons for the ‘hole’ the worldwide observed increases of CFCs detected throughout the atmosphere, and the unique wintertime meteorological environment over Antarctica. Between particular altitudes above Antarctica, the very cold stratospheric temperatures allow ice crystals clouds to form.
In these clouds, chlorine molecules from CFCs are chemically released during the darkness of the polar winter. The resultant build-up of chlorine molecules is reduced to ozone-destroying chlorine atoms by the action of UV when sunlight begins in September over Antarctica.
Global ozone decline
Ground-based and satellite measurements show significant decreases of total column ozone in winter and summer for both northern and southern hemispheres at middle and high latitudes. These downward trends were larger during the 1980s than in the 1970s. No statistically significant trends have been determined for the tropics during the 1980s. The most advanced computer models of global chemical destruction of stratospheric ozone explain most of the observed trend of total ozone in middle latitudes in summer, but only about half of that in winter. This means that future global ozone changes cannot be satisfactorily predicted as yet.
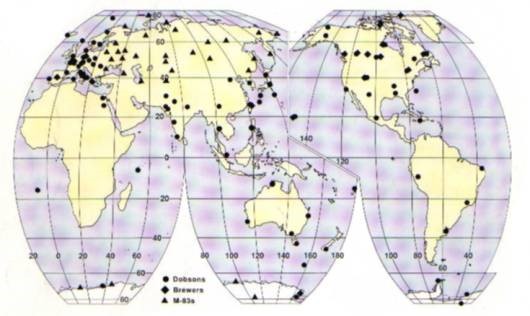
Measurement of Ozone
Total ozone measurements are conducted using three different platforms. These platforms include a surface based solar spectrophotometer, ozonesondes which reach stratospheric levels and near earth orbit satellite based sensors.
| Satellites |
The use of polar-orbiting satellites such as the Nimbus7 NASA Satellite carrying the Total Ozone Mapping Spectrometer (TOMS) instruments has revolutionized ozone monitoring over the past 20 years. Their high vantage point in space, and the ability of each satellite to traverse the entire globe, provides a much better coverage than ground stations. This is invaluable in determining global trends. The most accurate satellite sensors use the same principles as the Dobson spectrophotometer. |
| Dobson spectrophotometer |
The first spectrophotometer was built in the 1920s by Gordon Dobson for the purpose of measuring total ozone. Today about 80 of these types of instruments are used worldwide for measurement of total ozone. The Dobson spectrophotometer measures ozone by comparing the amount of sunlight at two ultraviolet lengths. One wavelength is strongly affected by ozone; the other is not. The difference between the two sunlight amounts is directly related to the total ozone. |
| Ozone sonde |
An ozone sonde is an electrochemical cell and radio transmitter attached to a hydrogen-filled balloon that can reach a height of about 35 km. Air is drawn into small cell by a pump. The solution in the cell reacts with ozone, producing a small electric current that is directly proportional to the ozone amount. The signal from the cell is converted into a code and transmitted via radio to a receiving station. From the launch of the balloon until its failure, typically at about 35 km, the sonde provides a vertical distribution of ozone. |

Spektrofotometer Dobson

Ozon Sonde
Action at the international level
In 1975, following concern that human activities may threaten the ozone layer, the WMO, at the request of the United Nations Environment Programme (UNEP) initiated the Global Ozone Research and Monitoring Project to coordinate the long-term monitoring and research of ozone.
All data from monitoring sites all over the world are transmitted to the World Ozone Data Centre in Toronto, Canada, where they are available to the international scientific community.
In 1977 a UNEP meeting of experts adopted the World Plan of Action on the Ozone Layer; in 1987 UNEP adopted the Montreal Protocol on Substances that Deplete the Ozone Layer.
The Protocol introduced a series of measures, including a timetable for action, to regulate the production and release of CFCs into the environment. It called for the levels of consumption and production of certain CFCs to return to 1986 levels by 1989, and a further 50% reduction by 1999. These measures are subject to further review.