Acid Deposition
Global environmental issues
The depletion of the stratospheric ozone layer, climate change, atmospheric acidification, desertification, loss of biodiversity and marine pollution are environmental issues of major concern reflecting the increasing influence of human activities on our fragile ecosystem. Why are they called global environmental issues? It is because their impacts and damages affect not only the countries where the problems originated, but go beyond political boundaries and can reach global scale.
Many of these problems are interrelated in a very complex manner. For instance, burning of fossil fuels emits carbon dioxide that contributes to global warming. At the same time it is also releases gaseous sulphur dioxide and nitrogen oxides into the atmosphere that are the major precursors of acid deposition. These problems may lead to soil degradation, forest die-back and threaten survival of our wildlife. As the world population continues to increase, pollution control presents a major challenge. Large amounts of resources and energy will be required in production to meet growing demands and huge quantities of wastes will be generated. This is one of the major causes of global environmental problems.
What is acid deposition?
The history of acidification began several hundred million years ago. Over time there lived and died plants and animals that were transformed into the material that we now refer to as fossil fuels such as coal, oil and natural gas. Over the past couple of hundred years we have been rapidly burning up these stores of organic material.
When we burn oil and coal from boilers in factories and power plants or burn fuel in our automobiles, we release into the atmosphere millions of tons of sulphur and nitrogen in the form of sulphur dioxide and nitrogen oxides. These pollutants can be transported over long distances by the wind, undergoing chemical transformation to form sulphuric and nitric acids which return to the ground as acid deposition.
Acid deposition is a term that includes any form of precipitation with acidic components. The manner in which these acidic components deposit to the ground determine whether it is a wet or dry deposition. Wet deposition is known as acid rain. Acid rain occurs when sulphur dioxide (SO2) and nitrogen oxides (NOx) emitted into the atmosphere react with water to form sulfuric and nitric acids which is then falls onto the ground.
Meanwhile, dry deposition occurs when gaseous substances such as sulphur dioxide, nitrogen dioxides and nitric acid deposited and stick to the surface of plants, soil and other materials in the absence of moisture.
Impact of acid deposition
There are many reasons why we consider acid deposition as one of the most serious environmental problems facing developed as well as developing countries today. How does acid deposition affect our ecosystem? In Europe and North America, the environmental effects are already well documented whereas in our region, there is a need for more studies focusing on the tropical ecosystem. Let us look at some of the impacts.
In the wet deposition process, sulphuric and nitric acids are incorporated into cloud droplets during cloud formation. These raindrops will eventually fall onto the ground in the form of rain and snow. When high concentrations of acid are present, the rain shows strong acidity.
Gaseous sulphur dioxide, nitrogen oxides and nitric acid, as well as acid aerosols are also deposited directly when they contact and adhere to the surface of vegetation, soil and other materials during fine weather. This process is known as dry deposition. In addition, the acids are attached to the particles suspended in the air and be deposited onto the surface of the land, buildings, plants and so on and thus the occurrence of dry acidic deposition.
Impak mendapan asid terhadap
In extreme cases, high levels of acid in the air cause skin problems and eye irritations. Chemical reactions occur between acids and alkaline substances in the atmosphere to form compounds which when inhaled, can affect human health. One of these compounds is ammonium sulphate, which is produced in large quantities during large-scale biomass burning events. When inhaled at high concentrations, these compounds are found to aggravate respiratory related problems in sensitive groups. The highly hygroscopic nature of ammonium sulphate allows it to absorb moisture under humid conditions resulting in a reduction of visibility or hazy conditions.
The biggest problem associated with acidic water is that it dissolves metals from both soil and water pipes. In Sweden, wells impacted by acidified groundwater contain more corrosive water with elevated levels of potentially harmful metals, particularly copper and aluminium. The effect from drinking the such acidified water is still a subject of debate among experts, but there is no doubt that consuming high levels of copper, iron, zinc and cadmium from pipes can be harmful to our health.
Acidification affects the ecosystem balance in lakes and rivers. Most species of aquatic animals, insects, plants and micro-organisms cannot tolerate large changes in acidity and their population are reduced when acidity increases. Eventually the species may be wiped out in much acidified waters. Aquatic insects, animals and plant plankton are amongst the most sensitive and they experience large decline in numbers in acid waters. Frogs and toads suffer reproduction disorders. On the other hand, mosses and some species of algae are known to thrive in acid waters.
Some sensitive fish species are unable to tolerate slight increase of acidity while others are killed due to aluminium poisoning, as a consequence of the acidification. In the Scandinavian countries several fish species including Atlantic salmon and brown trout, disappeared after rivers and lakes became acidic. Snow with acidic substances deposited during the very cold winter common in Scandinavian and North American countries and rapidly melts in spring. Salmon spawn in autumn and their fry normally remain in the rivers where they are born for about half a year until spring. Many salmon fry do not survive the acid surge associated with the snow-melting season.
Increasing acidification deprives the soil of its nutrient reserves as well as releases aluminium and certain heavy metals into the ground water, which when taken up by plants, causes damage. In the tropics, biomass burning associated with land clearing is widespread resulting in large quantities of sulphur dioxide and nitrogen oxides released into the atmosphere. Although the impacts on our forests have not been fully investigated, it is expected to increase leaching of tropical soils.
Scientific studies strongly indicate that air pollutants, directly or indirectly, causes forest damage at a regional level. Periods of drought and frost have a more severe and long-lasting impact on trees already exposed to air pollution. The extent of forest damage in Europe is a matter of very grave concern. For example, high sulphur coal from the Black Triangle near the borders of Germany, Czech and Poland was used as a fuel for power plants. Emissions of sulphur dioxide from industrial processes caused significant damage to their forests. A similar situation was observed in Chongqing, China.
Sulphur dioxide is emitted during the process of refining metals such as copper and nickel that contain high levels of sulphur. In the past, forest dieback in Ashio, Japan was due to high concentrations of sulphur dioxide generated from its copper refining industry. Even now, we see similar damage occurring in other parts of the world. For example, we can observe tens of kilometres of dead forests around the metal refining factories in Kora Peninsula in the western part of Russia.
Dirty air can cause irreplaceable damage to our historic and cultural monuments. If existing pollutant levels persist or are aggravated, deterioration of our historic buildings particularly those made of marble or stone, sculptures, monuments and artifacts will accelerate. Can we allow our ties with history to literally weather away before our eyes?
Sulphur dioxide affects the composition of leather, fabrics and paper causing significant deterioration. Corrosion of iron and steel structures is most extensive in polluted industrial sites.
Have you seen what appear to be icicles on walls and eaves of old buildings and highways? Dirty raindrops seep into walls through cracks, dissolve calcium in concrete materials and then leach out the walls. They combine with carbon dioxide in the air and form calcium carbonate, which grows like icicles. Wherever we observe these icicles, we can find dirty droplets at the top of the icicles.
How can we determine whether we have an acidification problem? This problem can be detected from measurements of our rainwater acidity. However, for a complete understanding of the problem we need to determine the contribution from both processes contributing to acidic deposition, namely wet and dry deposition.
A variety of instruments and techniques can be used to determine the total amount of acids that are deposited onto the earth. These instruments can range from very simple ones to very sophisticated systems that provide a high degree of accuracy. Similarly, assessment techniques can vary from very simple methods to the use of complex computer models that have the capability of predicting future scenarios.
Acidity of a liquid is a measure of its hydrogen ion concentration. It is normally expressed in terms of a pH value. On a pH scale of 0 to 14, a value of less than 7 is acidic, pH 7 is neutral and a pH value greater than 7 is alkaline.
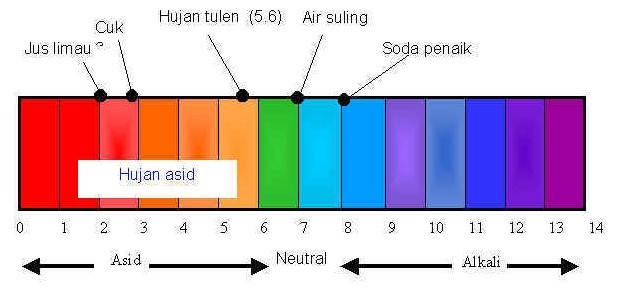
Acidic rain measurement starts with the collection of rainwater. The collection of rainwater should be done in a well exposed location far from obstructions such as buildings and trees. In urban areas, we should avoid sites that are significantly affected by local emissions such as those from automobiles and industrial boilers. Care must be taken to ensure that the rainwater samples are not contaminated during the collection process. Avoid any form of contact with your hands. The collector should first be cleaned thoroughly with high purity water before use. Glass or plastic collectors are preferred since they will not react with the rainwater. The pH of rainwater is measured either by using pH indicator strips and a pH colour chart, or by using a pH meter.
Although pH is an important indicator of acidity, the relationship between pH value and acid concentration is rather complex as it is not linear. pH values depend on the balance of acid and base in the solution. A good approach for understanding the acidification of rainwater is to determine the concentrations of sulphate and nitrate ions which is a more comprehensive approach. Automatic tool is used to collect rainwater samples. Rainfall amount, duration and expiry are also recorded. This information is used together with the results of chemical analysis of the presence of ions in rainwater during the evaluation of acid deposition.
Dry deposition is less well studied than wet deposition because it is more difficult to quantify. However, it is of equal importance to wet deposition.
Passive samplers, filter packs or gas analyzers can be used to measure concentrations of acidic gases such as sulphur dioxide, oxides of nitrogen as well as other important gases such as ammonia that contribute to the acidification process. Filter packs are easy to operate and are widely used at both urban and remote sites. However, initial preparation of the filter needs to be done and the operation requires electrical power.
Both these methods require initial preparation of the filters and final analysis of the exposed filters in a chemical laboratory. The active analyzers, on the other hand, provide continuous, real-time values of gas concentrations, but they are more difficult to maintain.
Dry deposition of acidic aerosols are usually less important than dry deposition of gas as the amount of acid in the aerosol deposition is less than in the form of gas. Aerosol samples are collected on the filter by using the low volume air sampler tool and then are analyzed to determine the concentration of the acid.
Acid deposition monitoring network in Malaysia
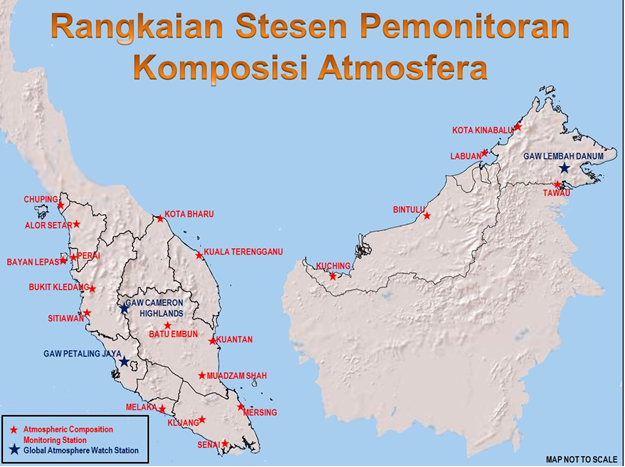
The Malaysian acid deposition monitoring network comprises of 22 stations of which 17 are located in Peninsular Malaysia, 4 stations in Sabah and 2 stations in Sarawak. All these stations are equipped with automatic wet-only samplers to collect rainwater samples over a prescribed period of time. These samples are then chemically analyzed for the following elements: calcium, lead, manganese, iron, nickel, sodium, copper, magnesium, zinc, mercury, potassium, sulphate, nitrate, chloride, fluoride and ammonium. The conductivity and pH of the rainwater is also measured.
Sources of acidic pollutants
The main cause of air pollution can be categorized into mobile (mobile vehicle) and stationary sources (manufacturing activities). In addition, another source of acidic pollutants is the open burning. Motor vehicles are the principal contributor of acidic pollutants among the mobile sources. Transportation is expected to remain a major contributor in the future as the population of vehicles is expected to increase.
Solutions and counter measures
Industry can take several actions to alleviate the problems of acid deposition. The best solution is to limit the emission of pollutants at their source. Emissions from industrial activities can be reduced by enforcing regulations, fuel gas treatment, control technologies and careful environmental planning.
Industries can limit their emission of acidic pollutants through two methods. They can switch to using fuels that have no sulphur or low sulphur content. To reduce the sulphur component in oil, petroleum companies should use desulfurization during the refining process. Currently most of the fuels used around the world have sulphur content less than 0.03%. By reducing the sulphur content in oil, all activities that use oil in their operations will emit lower quantities acidic pollutants.
Some industries install scrubbers in their smokestacks to reduce the amount of sulphur dioxide released into the atmosphere. Factories that use fuels with high sulphur content should install flue gas desulfurization systems. The installation of denitrification and desulfurization facilities can significantly reduce the nitrogen oxide and sulphur oxide emissions. Strengthening existing regulations and emissions standards can help to enforce the installation of these facilities.
The reduction of nitrogen oxides from mobile sources is more difficult to address as they are primarily emitted from automobile exhaust. However they can be controlled by regulating the use of specially designed catalytic converters. Reduction of nitrogen oxide emissions can also be achieved by improving combustion efficiency.
Besides the use of control technologies, the Malaysian government has also implemented several laws to control the emission of air pollution. Under the Environmental Quality Act 1974 (Amendment 1996), there are several regulations to control air pollution such as:
Environmental Quality (Clean Air) Regulations 1978
Environmental Quality (Control of Emissions from Diesel Engines) Regulations 1996
Environmental Quality (Control of Emissions from Petrol Engines) Regulations 1996
Some countries have set very stringent limits to emissions. Emission standards are normally set based on either of the following:
To achieve a certain reduction of emissions, assuming that the technology to achieve the required efficiency will be developed; and
To choose a suitable available technology and set emissions standards at a level that technology can achieve.
How can we improve the environment?
Do not consume energy unless you need it. Avoid over-cooling rooms, leaving lights and electrical items such as television sets and fans switched on in empty rooms. Avoid using electrical devices that do not operate efficiently.
Reduce activities that involve burning fossil fuels. The burning of fossil fuels will emit gases such as carbon dioxide, carbon monoxide, sulphur dioxide and oxides of nitrogen as well as particulates and toxic metals that can pollute our atmosphere and affect our health and quality of life.
Ensure that your vehicle is in good condition and is regularly maintained. Vehicle owners should always ensure that their vehicles do not emit excessive amount of smoke. Malaysia presently has more than 10 million registered vehicles. If each vehicle is well maintained, then emissions from vehicle exhaust can be minimized.
Choose the most energy saving and least polluting mode of transport. Use mass transportation such as trains or buses, or even better, bicycles or car pool whenever possible.
Do not carry out open burning indiscriminately. All burning activities should be carried out in accordance with existing rules and regulations and each fire should be carefully guarded to prevent it from spreading out of control.
Develop an attitude of caring for the environment. Members of the public should do their part in maintaining cleanliness by not littering at all times. Avoid the selfish attitude of Not in My Backyard (NIMBY).





